In light of the Ebola cases seen in Texas, Nebraska and now New York, hospitals in the U.S. are reviewing their infectious disease preparedness plans. The Centers for Disease Control and Prevention (CDC) recommends risk assessments are conducted by each laboratory director, biosafety officer or other responsible personnel to determine exposure risk and course of action that best fits the facility. For the laboratory, this includes procuring dedicated equipment (preferably disposable) that can be used for routine laboratory testing of infectious patients separate from those used in the main lab.
Dedicated bedside lab instruments
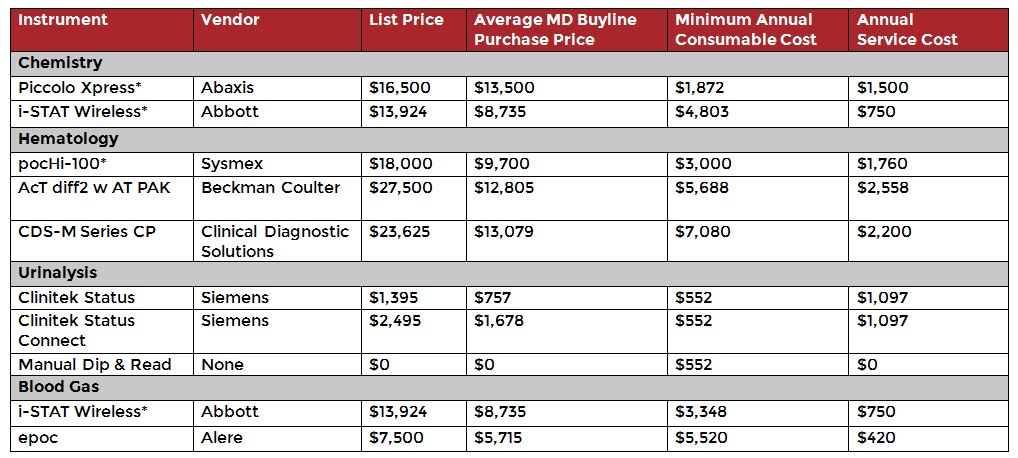
Since the lab is a highly regulated area of the hospital, shifting routine testing from the lab to the bedside will require equipment that can meet those same regulatory requirements. MD Buyline has gathered instrument recommendations and pricing for systems that meet these needs in the table below. These point of care (POC) instruments offer ease of use and portability for bedside testing, allowing testing to be performed by non-lab personnel. This eliminates the need to send routine tests (e.g., Complete Blood Count, Chemistry Panel) to the main laboratory, minimizes potential infectious disease exposure to lab personnel and reduces laboratory downtime for infectious disease cleaning and maintenance. POC testing can also be set up in a confined space, such as a containment area. The low cost of these instruments allows them to be disposal at end of use.
In the table, the asterisk identifies the top instrument choices for the various disciplines. These were selected based on their ease of use, quality of results, cost to purchase and maintain and portability. They are also the disposable systems of choice by top hospitals. Please note, this list is not meant to be exhaustive and includes optional instruments to meet the various needs of a hospital. Consumable considerations for all instruments can vary based on the volume and type of testing done.
The i-STAT in particular is unique in that it is a multi-function POC instrument that can run limited chemistry, blood gas, coagulation and hematological parameters.
Dedicated Laboratory Equipment Recommendations & Pricing
Source: MD Buyline
When considering bedside or POC testing, laboratory personnel should consider the waived testing options that are available. These offer less stringent accreditation requirements compared to those that are non-waived and meet the following criteria:
- Simple laboratory procedures
- Insignificant risk of erroneous result
- FDA approved for home use
- Pose no reasonable harm if performed incorrectly
Performing tests at the bedside does not exclude the instruments from having to meet regulatory requirements, nor does it include testing for the Ebola virus which is only performed by the CDC. The recommendation is solely to provide hospitals preparing for infectious disease treatment with options for dedicated instrumentation that can meet their immediate needs to test infected patient blood and bypass the main laboratory process.
Instrument procurement recommendations
A direct purchase may be the best option for infectious disease preparedness. An agreement such as lease, reagent rental or cost per test may not be the best option for this situation as the likelihood of prolonged testing and use is minimal. Minimizing extended costs will make the instrument more disposable than committing to a long-term agreement. Vendors may be able to provide free samples of some of the equipment for a limited amount of testing at the time of purchase.
Consumable agreements can be for a 12-month term rather than the standard five years. In the event that an infectious patient is admitted, additional products can be ordered at that time to meet the demands. Keep in mind that some instruments require daily start-up and shut-down procedures that will need reagents to function.
For facilities that already have these instruments in use, we recommend shifting older instruments for the infectious disease involvement and using newly purchased equipment for daily use. Discuss what options are available with vendors to shift service and reagent agreements already in place with older equipment to find extra savings. A service agreement past the 12-month warranty may not be necessary. Some instruments listed come with a 36-month warranty, negating the need to purchase additional service. Additional reagent and service needs can always be revisited at the end of the contract, although we always recommend negotiating reagent and service needs at the time of purchase even if they are to be purchased after contract expiration.
Be sure to have a laboratory technical procedure or Standard Operating Procedure (SOP) for each dedicated instrument available at the testing site. If an existing technical procedure is not available, vendors may be able to provide a sample procedure that can be customized and used by testing personnel. The procedures may include appropriate infectious disease warnings for non-lab testing personnel. Non-lab personnel performing testing on dedicated instruments should be trained in the proper use and maintenance of these instruments by a qualified individual. The technical procedure should be readily available in the event that questions arise, but it should not be a substitute for hands-on training.
Funding & grant opportunities
On October 16, the House Energy and Commerce Subcommittee on Oversight and Investigations held a hearing in which members expressed concern that budget cuts to the CDC and National Institutes of Health (NIH) as well as other public health programs hampered the nation's progress in stopping the disease and impacted the development of an Ebola vaccine. Rep. Peter Welch requested a hearing for the subcommittee to focus on the funding needs of the NIH and CDC, as well as the nation's infrastructure. It was noted by Chairman Murphy that the subcommittee will hold a follow-up hearing in November 2014, although no specifics were provided on the topics of discussion.
Historically, the Hospital Preparedness Program (HPP) and Public Health Emergency Preparedness provide funding through grants and cooperative agreements to states, territories and eligible municipalities to improve surge capacity and enhance community and hospital preparedness in the event of a public health emergency. The U.S. Department of Health and Human Services', Office of the Assistant Secretary for Preparedness and Response and CDC provide instructions for Preparing an Interim Progress Report, which outlines the eligibility and application submission process. Facilities seeking funding from the state or federal programs may need to provide pricing information for cost-based or charge-based reimbursement for instruments used in infectious disease preparedness. More information on grants can be found here.
It is unknown at this time how much (if any) reimbursement will be available for hospitals participating in Ebola preparedness. In the past, the HPP along with state and local health departments have purchased healthcare facility based equipment and supplies, trained and collaborated to address situations like Ebola and other infectious diseases. In the meantime, hospitals can focus on preparedness logistics until the details on funding availability become clear.
Moving forward
Every department in the hospital will face unique challenges in establishing and managing preparedness for possible infectious disease cases. In the laboratory setting, providers will need to determine if they want to continue current processes which would include routing testing through the laboratory and utilizing either primary or backup equipment or if they want to test at the beside. In either case, laboratory personnel should stay informed on current CDC recommendations for evaluating Ebola patients in the lab and managing the continued care of patients with confirmed cases.
More information:
Cheat sheet of the CDC Guidance on Ebola in the Lab, created by MD Buyline
The views, opinions and positions expressed within these guest posts are those of the author alone and do not represent those of Becker's Hospital Review/Becker's Healthcare. The accuracy, completeness and validity of any statements made within this article are not guaranteed. We accept no liability for any errors, omissions or representations. The copyright of this content belongs to the author and any liability with regards to infringement of intellectual property rights remains with them.