The concepts of patient engagement and shared decision-making may not be entirely new, but the Centers for Medicare & Medicaid Services (CMS) has recently ramped up its focus on these initiatives.
A few months ago it unveiled its Shared Decision-Making Model for accountable care organizations (ACOs) and it’s Direct Decision Support Model for decision support organizations (DSOs). Now, the agency is proposing a new quality measure as part of the Hospital Inpatient Quality Reporting (Hospital IQR) Program that emphasizes the vital role that comprehensive informed consent forms play in patient satisfaction.
For quite some time CMS’ Hospital Interpretive Guidelines have offered significant benchmarks for a “well-designed” consent form and process—including the provision of procedure- and patient-specific information and assessment of patient perceptions of the process. The belief is that a comprehensive informed consent discussion can improve patient satisfaction and care decisions by better aligning patients’ treatment expectations with their preferences and goals.
The proposed Hospital IQR Program quality measure now gives hospitals financial incentive to prove that theory—and advance patient-centered decision-making—by testing themselves against many of those best practices.
Subtle change, significant impact
Despite the importance of informed consent documents, the proposed CMS quality measure (Federal Register. 2017;82(81):20052-20055.) states that the documents frequently lack relevant procedure-specific information. In addition, those documents often are presented to patients minutes before the start of their procedures—a point at which CMS notes patients “are the most vulnerable and least likely to ask questions.”
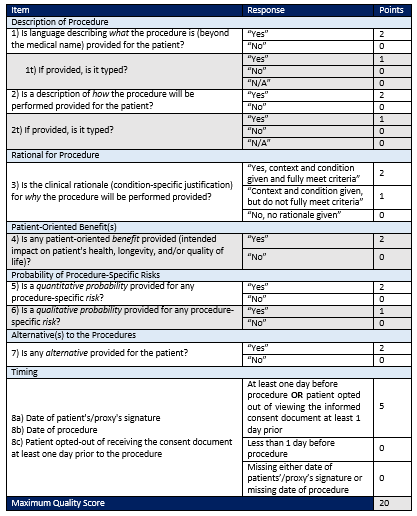
Consequently, at the heart of the proposed “Measure of Quality of Informed Consent Documents for Hospital-Performed, Elective Procedures” is an abstraction tool providers can use to evaluate eight specific “best-practice” elements. It’s essentially a scorecard that yields a maximum quality score of 20.
One very interesting note: CMS developed the abstraction tool in part using informed consents from eight hospitals. The agency then validated the tool by testing it in 25 additional hospitals. The facilities determined that the information generated helped identify important gaps in existing documentation, and as a result led to improvements in the informed consent process.
This level of significant effort and research for a single measure is rather unusual. In addition, preliminary feedback from purchaser and consumer organizations such as the Leapfrog Group applaud the measure and, if anything, suggest additions to further enhance the measure. For hospitals, this is a strong signal that the proposed measure is likely to be adopted with few changes. The good news is that taken together, the abstraction tool and the proposed measure give hospitals an easy-to-follow blueprint.
Steps to take now
Hospitals that follow the CMS guidance for a well-designed informed consent process should be well prepared for the new quality measure if it is adopted in its current form. Regardless of the level of adherence to existing guidelines, hospitals and health systems can start to prepare in these ways:
• Score yourself. The new abstraction tool makes it relatively straightforward for organizations to test a representative sample of their consent forms (see figure 1). For example, the first element asks, “Is language describing what the procedure is (beyond the medical name) provided for the patient?” A positive response yields two points toward the tool’s maximum quality score of 20. A “no” answer generates zero points.
Hospitals can use low scoring elements to identify which of their forms, policies or processes might need to be adjusted. For example, ensure that procedure-specific details (such as description, rationale for the procedure, benefits, risks and alternatives) are typed on the consent form, not handwritten.
• Evaluate timing. Along with signatures, CMS currently requires consent forms to include both the date and time of day they were signed. It’s relatively easy for CMS surveyors to determine if a consent form was in place at the time of surgery simply by reviewing the time of day the patient signed the form and the case start time.
Under the proposed measure, the patient’s signature must be obtained at least one day before an elective procedure or it must be noted that the patient opted out of viewing the informed consent document at least one day prior. CMS’ emphasis on the critical nature of consent timing is evident; the timing element represents 25 percent of the maximum quality score. In addition, it’s an all-or-nothing requirement. Hospitals with consent documentation in place at least a day prior to surgery score all five points. Those with less than a day—or missing dates—score zero points.
• Interview patients. In conjunction with the abstraction tool scores, organizations should also survey patients about their satisfaction with the consent process. CMS surveyors are instructed to do this today, so there is no reason a hospital should not proactively gather the same information. (If better satisfaction scores are aligned with higher abstraction tool scores, it might even help validate the consideration of making new investments to modify and improve the consent process.)
• Consolidate workflows. Physicians typically have consent discussions with their patients in their offices days or weeks prior to a procedure. Yet a disconnect occurs when that discussion is not captured on a hospital’s consent form. This gap can be closed by automating the consent process. Automation also allows hospital consents to be electronically appended to, and executed along with, the practice consent, thus empowering organizations to seamlessly meet the CMS requirement to execute the consent a day or more prior to the procedure.
If the proposed quality measure isn’t enough reason for hospitals to take a deeper look at their consent processes, liability costs and operational efficiency may be additional factors to consider. The timing of consent documentation is particularly relevant in these situations as well.
In medical malpractice cases alleging inadequate informed consent, consents obtained in the pre-operative holding area resulted in significantly higher legal expenses and indemnity payouts — an average of $322,000 higher — than cases where informed consent was obtained in the practice setting, according to a comprehensive analysis in the Journal of Bone and Joint Surgery. Such incremental financial liability and risk may be mitigated by obtaining informed consent in the physician’s office.
Research published in JAMA Surgery has found that informed consent documents are frequently missing at the time of surgery resulting in OR start time delays in 14 percent of cases. Those costly delays may be eliminated if consent is documented the day before – preferably using an automated system that places the document in the electronic health record (EHR) for ready access during the pre-procedure verification and/or during the time-out.
A rare easy win
The proposed CMS Quality of Informed Consent Documents measure is a needed step toward ensuring patient engagement and shared decision-making. Like other Hospital IQR Program measures, if approved, the rating of the informed consent process will likely be documented on the Hospital Compare website. Beyond contributing to an organization’s star rating, this measure would resonate as perhaps the strongest indicator of how effectively providers communicate with patients.
In terms of when to invest in maximizing an organization’s score on the proposed measure, forward-thinking organizations will begin that effort immediately. There is virtually no downside from a risk, safety or satisfaction standpoint to cultivating patients who are better informed, can describe what is going to happen to them, can understand their available treatment alternatives, and who have reasonable expectations about outcomes and potential complications.
At a high level, the proposed measure supports the Triple Aim of better care, smarter spending and healthier people. It’s an easy, manageable win for hospitals and health systems that proactively prepare for what is likely to be a new standard of quality.
About the author
Tim Kelly, MS, MBA is director at Taylor Healthcare.
Figure 1 – Abstraction Tool for Informed Consent Form Scoring
The views, opinions and positions expressed within these guest posts are those of the author alone and do not represent those of Becker's Hospital Review/Becker's Healthcare. The accuracy, completeness and validity of any statements made within this article are not guaranteed. We accept no liability for any errors, omissions or representations. The copyright of this content belongs to the author and any liability with regards to infringement of intellectual property rights remains with them.